Descriptions for special techniques we are using:
- 2Psetup
- Open-source software for zebrafish eye tracking and visual stimulation
- Transgenic Zebrafish
- Visual stimulation setup
2P setup
In the Arrenberg lab we perform two-photon calcium imaging with a Coherent Chameleon Vision S laser and a Sutter Instrument Movable Objective Microscope (MOM, Euler et al. 2009, https://sutter.com/MICROSCOPES/index.html ). As we are interested in different animal behaviors and aspects of visual processing, we make use of various experimental techniques in combination with 2P imaging to elucidate neuronal circuits and behavior.
While we use a simple custom-made LED arena to display vertical gratings around the fish to elicit eye and tail movements, and an IR (high speed)-camera to record them, we additionally employ cylindrical and spherical LED arenas – with almost 360° coverage – for more elaborate stimulations (e.g. optic flow, receptive field estimation).
We also developed fiber optic-based and DMD-based optogenetic photostimulation setups, which we can use with different transgenic animal lines to spatially activate or silence specific subsets of neurons. Together with microinjection and electroporation tools we have various additional approaches to use in tandem with calcium imaging. Additionally, we are in the process of setting up a 2nd microscope with an additional rotation axis and long wavelength multiphoton stimulation to make use of the better tissue penetration and potentially of RCaMPs (red calcium indicator) in the future.
ZebEyetrack: Open-source software for zebrafish eye tracking and visual stimulation
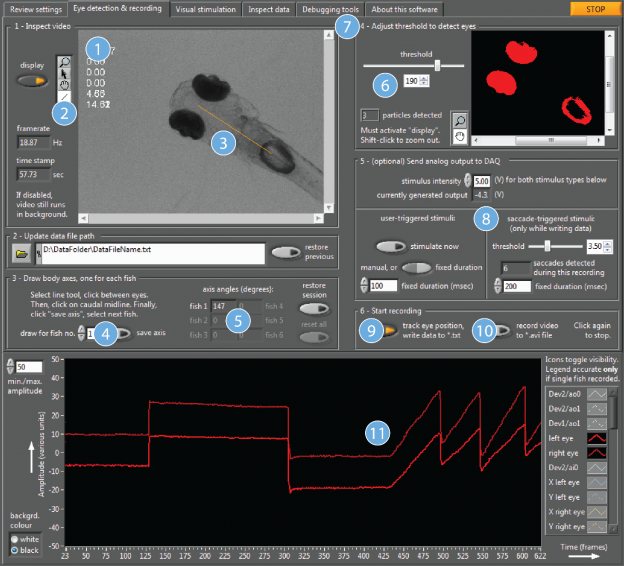
The optokinetic response, or OKR is a very commonly used and robust method of assessing visual function and evoking eye movements in zebrafish larvae. The OKR is a reflex allowing the visual environment to be stabilised on the retina. It consists of slow velocity phases where the eye “follows” the movement of the entire visual field, and fast resetting saccadic eye movements, usually evoked when the eye has reached the periphery and can no longer follow the stimulus. This reflex is readily elicited by a grating stimulus consisting of vertical moving bars. In our lab, we investigate the oculomotor hindbrain circuits controlling horizontal eye movements during optokinetic and spontaneous eye movements. To make this easier, we have developed a software which integrates all key aspects of behavioural vision experimentation, including stimulus design, visual stimulation with moving bars, eye detection and tracking (and general motion detection), real-time analysis, eye position dependent closed-loop event control and recording of external event times.
ZebEyeTrack and its precursor version have already been used in many applications, including: (i) spontaneous eye movements tracking, (ii) event control for optogenetics, (iii) calcium imaging, (iv) event control for calcium imaging and (v) student training.
This software has an intuitive graphic user interface, and can be easily integrated into existing setups. We are committed to sharing this program with scientists who need to test zebrafish visual performance in their projects. The software can be downloaded for free from our dedicated website http://www.zebeyetrack.com/. You can also test our software or analyse your own data by using the ZebEyeTrack virtual machine that we host (no download is needed, instructions are available at www.zebeyetrack.com). Also see Dehmelt et al. Nature Protocols 2018 for a step-by-step introduction to this software.
(Gonçalves et al. Frontiers in Neural Circuits 2014)
The animal performed two spontaneous saccades, after which the visual stimulus was turned on and the animal started to perform the optokinetic response (red lines: angular eye position traces over time).
Transgenic Zebrafish
Generating new transgenic lines is an ongoing focus of the lab, allowing us to investigate and manipulate increasingly more specific neuronal populations. In our fish room we have available a range of different zebrafish lines, which allow us to, for example, label distinct neuronal cell types, observe the effects of mutations, visualise calcium activity, and optogenetically manipulate neuronal activity. Most of the lines are generated by plasmid microinjection at the one cell stage in the laboratories of collaborators or in our own laboratory. We use a variety of different genetic techniques to generate the needed zebrafish lines, with the most common approach being a combination of Tol2 mediated insertion of DNA into the genome and the Gal4-UAS system.
The Gal4-UAS system is a flexible and efficient way to quickly generate many zebrafish with novel properties through the use of a small number of starter lines. Briefly, a Gal4 expressing promoter line must be crossed with a UAS containing reporter line in order for expression to occur. Once established, maintaining a diverse range of Gal4 promoter lines and UAS reporter lines – which can be crossed to generate the lines needed for an experiment – takes less time than that needed to inject a full plasmid containing both inserts into individual embryos, raise these embryos to adulthood (F0) and use their offspring (F1).
Visual stimulation setup
Zebrafish have laterally located eyes and therefore a large field of view. This means that for an effective stimulation of visual brain areas we need to cover a comparably large area (almost the whole surround).
We currently use two different kinds of visual stimulation setups. One of which offers a 80° x 330° (elevation x azimuth) cylindrical coverage at a high temporal and spatial resolution (approx. 1.5°) using individually controllable LEDs. The fish are mounted in the center of a water-filled glass bulb, which minimizes distortions and reflections of the visual stimuli. The second one covers a spherical area of 180° x 330° using the same LED based system.
In both setups we can simultaneously record neuronal activity with two-photon calcium imaging and fish behavior (eye and tail movements) during visual stimulation by the LEDs.
Additionally, we are in the process of constructing an entirely new setup which utilizes a video projector (digital micromirror array combined with LEDs) to create an immersive 240° x 360° multi-color visual environment for the fish and which will allow us to also simultaneously record neuronal activity and fish behavior. This will enable us to create naturalistic visual underwater scenes while studying the fish’s responses.



